Development of Grade 10 Students’ Conceptual Understanding of Dot Structure and Molecular Shape from Learning by Using Magnet and Pin Kits
Main Article Content
Abstract
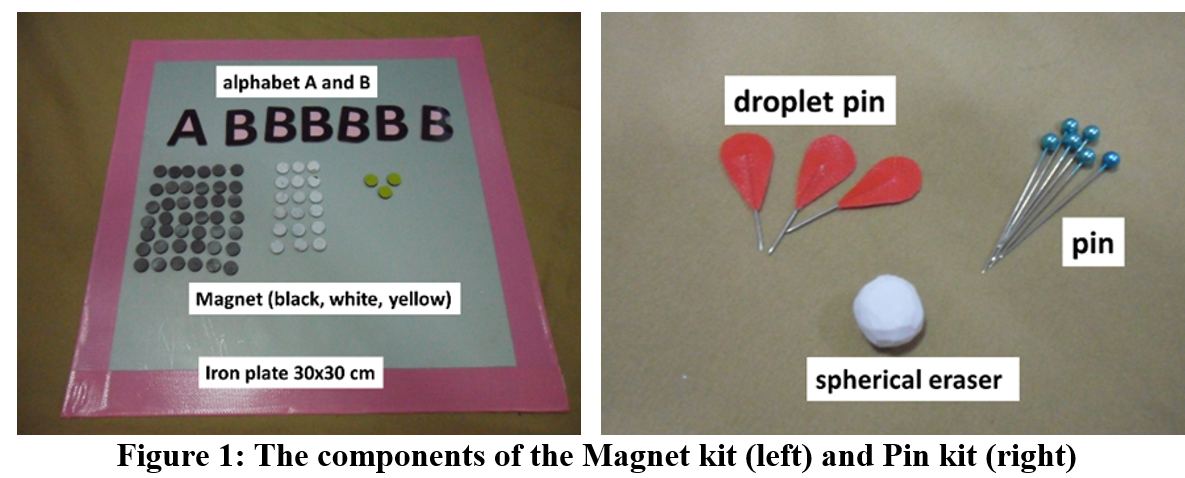
This research aims to develop grade 10 students’ conceptual understanding of the topic of dot structure and molecular shape from learning by using the Magnet and Pin kits. The research methodology is one group pretest-posttest design. The participants are 33 grade 10 students at the Demonstration School of Khon Kaen University (Thailand), acquired by purposive sampling. The data was collected using the Dot structure and Molecular shape Conceptual Test (DMCT). The DMCT will be used to collect data for the pre-test and post-test. There are six items in the DMCT (difference in six molecular formulas; PF5, ClO4-, CO32-, O3, ClF3 and XeOF4 ). The answers form DMCT were analyzed by classifying the conceptual understanding at 5 levels according to the conceptual framework of Ҫalik, Ayas, and Coll (2009). The results found that before learning, most of the students had a conceptual understanding at the NU level of the ClO4-, CO32- ,O3, ClF3, XeOF4 molecule and had SAC level understanding of the PF5 molecule. It showed that the students’ conceptual understanding of all molecules was very low. After learning with the Magnet and Pin kits, most of the students’ conceptual understanding level increased to the SU level for the PF5, CO32-, ClF3 and XeOF4 molecules and increased to the PU level for the O3 and ClO4- molecule. This shows that the use of Magnet and Pin kits in learning to draw the Lewis dot structure and to make molecular shape prediction can help students achieve an overall higher level of conception from NU and SAC to SU and PU levels. The students could specify the type and number of central atoms, surrounding atoms, number of bond pair electrons, and lone pair electrons. The students could also draw the Lewis dot structure correctly and they could draw images to represent three-dimensional shapes of molecules and correctly specify the names of molecular shapes.
Article Details

This work is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License.
References
Ahmad, W. Y., & Omar. S. (1992). Drawing Lewis structure: a step-by-step approach. Journal of Chemical Education, 69(10): 791-792.
Ahmad, W. Y., & Zakaria, M. B. (2000). Drawing Lewis structure from Lewis symbols: a direct electron pairing approach. Journal of Chemical Education, 77(3): 329-331.
Çalik, M., Ayas, A., & Coll, R. K. (2009). Investigating the effectiveness of an analogy activity in improving students’ conceptual change for solution chemistry concepts. International Journal of Science and Mathematics Education, 7: 651-676.
Chang, R. (2010). Chemistry. McGraw-Hill, USA.
Coll, R.K., France, B. & Taylor, I. (2005). The role of models/and analogies in science education: Implications from research. International Journal of Science Education, 27 (2): 183-198.
Kamkhou, K., & Yuenyong, C. (2019). Magnet and Pin kit: connection symbolic and submicroscopic representations of Lewis dot structure and molecular geometry. Journal
of Physics: Conference Series, 1340(1): 012070.
Kimball, D. B. (2012). Adaptive instructional aids for teaching a blind student in a nonmajors college chemistry course. Journal of Chemical Education, 89: 1395-1399.
Kiste, A. L., Hooper, R. G., Scott, G. E., & Bush, S. D. (2016). Atomic tiles: manipulative resources for exploring bonding and molecular structure. Journal of Chemical Education, 93: 1900-1903.
Pardo, J. Q. (1989). Teaching a Model for Writing Lewis Structures. Journal of Chemical Education, 66(6): 456-458.
Sunson, P., & Wuttisela, K. (2015). Development of grade 10 students’ science concepts of covalent molecular shapes through Model-Observe-Reflect-Explain (MORE). Humanity and Social Science Journal Ubon Ratchathani University, 6(2): 83-97.
Supatchaiyawong, P., Faikhamta, C., & Suwanruji, P. (2015). Using model-based learning for enhancing mental model of atomic structure and understandings of the nature of model of 10th grade students. Journal of Education and Innovative Learning, 1(1): 97-124.
Tan, K. C. D., & Yeo, J. (2022). Advancing Conceptual Understanding of Science. International Journal of Science Education and Teaching, 1(2): 56-64.
Turner, K. L. (2016). A cost-effective physical modelling exercise to develop student’s understanding of covalent bonding. Journal of Chemical Education, 93: 1073-1080.
Udomkan, W., Suwannoi, P., Chanpeng, P., Yuenyong, C. (2015). Thai Pre-service Chemistry Teachers’ Constructivist Teaching Performances. Mediterranean Journal of Social Sciences, 6(4 S3): 223-232.
Urasin, S., & Supasorn, S. (2011). Comparing students’ conceptions of chemical bonds prior and after the implementation of paper-based T5 learning model. KKU Research Journal, 1(1): 38-57.
Wichaidit, P. R. (2015). Nature of chemistry and performing an instruction to be consistent with its nature. Srinakharinwirot Science Journal, 31(2): 187-199.
Wuttisela, K. (2014). An alternative molecular model for teaching valence shell electron pair repulsion theory. Journal of Research Unit on Science, Technology and Environment for Learning, 5(2): 209-213.
Yuenyong, C., & Thathong, K. (2015). Physics teachers’ constructing knowledge base for physics teaching regarding constructivism in Thai contexts. Mediterranean Journal of Social Sciences, 6(2): 546-553.
Zandler, M. E., & Talaty, E. R. (1984). The “6N+2 Rule” for writing Lewis octet structure. Journal of Chemical Education, 61(2): 124-127.